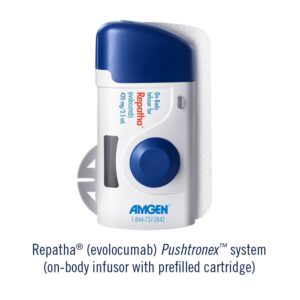
Amgen announced that the U.S. Food and Drug Administration (FDA) has approved the Repatha (evolocumab) Pushtronex™ system (on-body infusor with prefilled cartridge), a new, monthly single-dose administration option.1 The Pushtronex system is a hands-free device designed to provide 420 mg of Repatha in a single dose.
Continue Reading Article
News provided by Amgen on 7/11/16